

Imagine you’re at a bustling party where everyone is milling about randomly—no one’s dancing together. Now, picture a friend rolling out a long, narrow carpet and suddenly, people line up and start waltzing in pairs. That’s a bit like what happened on early Earth, only the ‘carpet’ was a mineral called α‑alumina, and the dancers were the building blocks of life.
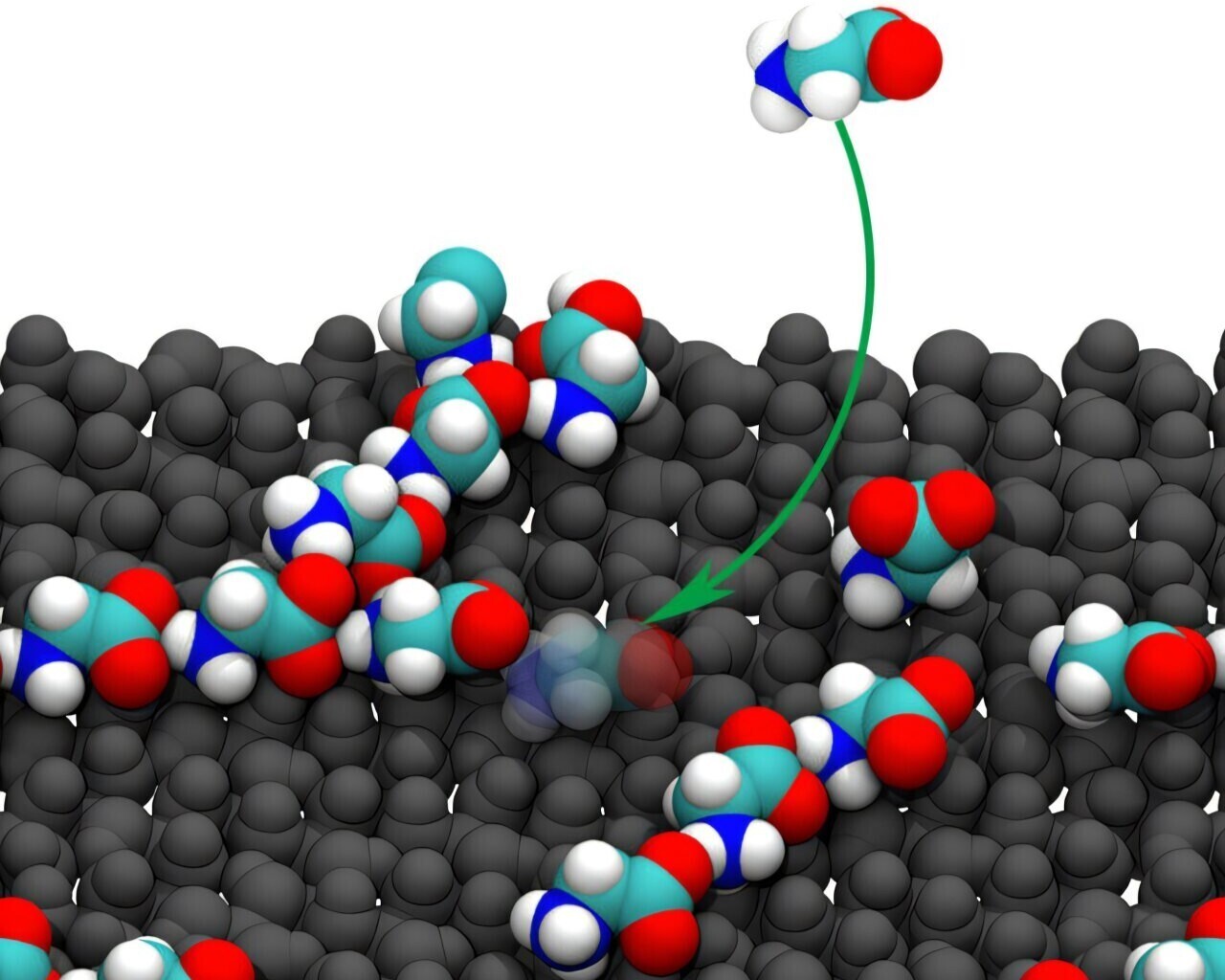
α‑Alumina (a form of aluminium oxide) is one of the most common minerals in Earth’s crust — think of it as the ‘everyday rock’ hiding beneath our feet and in clay, sand, and even the gritty surface of your kitchen countertop. Scientists have now shown that its flat, orderly surfaces could have served as nature’s own dance floor for simple molecules.
The big mystery has always been how lone amino acids, tiny molecules like glycine, which can drift aimlessly in water, ever managed to snap together into long chains. These chains, or polymers, are the precursors to proteins, the workhorses of every living cell. Using advanced computer simulations, researchers discovered that glycine molecules are irresistibly drawn to the α‑alumina surface and neatly line up along its atomic grooves—much like beads clicking into a string. Even more astonishing, this mineral ‘template’ boosted the odds of forming chains of ten or more glycine units by over 100,000 times compared to when the amino acids floated freely in the water.
Water usually cloaks amino acids in a shell of molecules, making it hard for them to bond. But the α‑alumina surface seems to gently pry away these water shells, holding glycine molecules in just the right orientation and distance to encourage chemical handshakes. In effect, the mineral does the heavy lifting and aligning the molecules, squeezing out excess water, and giving them the perfect conditions to click together.
Why does this matter? By revealing how an ordinary rock could have kick‑started the assembly of life’s first molecular chains, we’re one step closer to solving the riddle of how non‑living chemistry blossomed into living systems. And beyond peering into our ancient past, these insights could inspire new ‘biomimetic’ materials, artificial surfaces that mimic α‑alumina’s talent for organising molecules. Imagine smarter drug‑delivery platforms, greener catalysts, or even lab‑grown tissues guided by mineral scaffolds.
In short, the next time you brush against a ceramic tile or spot a lump of clay, remember — Earth’s humble minerals may have been the unsung heroes that turned simple molecules into the very fabric of life.
Image and information credit: https://phys.org/



Responses






