

Researchers from Tokyo Metropolitan University (TMU), a public research university in Hachioji, Tokyo, Japan, have developed cutting-edge nanostructured alumina surfaces that deliver remarkable antibacterial resistance while promoting the healthy growth of cultured cells. The team used advanced electrochemical techniques with concentrated sulfuric acid to create anodic porous alumina (APA) surfaces with exceptional antibacterial properties. These innovative surfaces effectively prevent bacterial growth without compromising cell cultures, paving the way for safer and antibiotic-free cell culture environments. This breakthrough technology holds the potential to revolutionise regenerative medicine by enabling the production of high-quality, contamination-free cell cultures.
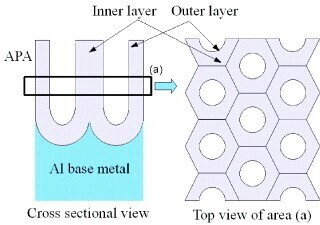
Antibacterial surfaces play a vital role in safeguarding public health and improving daily life. Traditional approaches, such as using antibiotics and harsh chemicals, often come with significant downsides, including environmental harm, health risks, and the growing issue of antibiotic-resistant bacteria. As these challenges mount, the demand for innovative, eco-friendly solutions to combat bacterial pathogens is more pressing than ever. Nanostructured surfaces, like anodic porous alumina (APA), offer a promising and sustainable alternative.
The concept of nanostructured surfaces as antibacterial agents has its roots in nature. In the early 2010s, researchers discovered that the naturally occurring nanostructures on cicada and dragonfly wings are highly effective at resisting bacterial contamination. These intricate surface patterns physically disrupt bacterial cell membranes, preventing their spread. Inspired by this remarkable natural defence, scientists have been exploring cost-effective methods to replicate such surfaces artificially, paving the way for a new era of safer and more sustainable antibacterial technologies.
Image credit: Tokyo Metropolitan University
Cell-friendly and antibacterial surfaces breakthrough
A research team led by Professor Takashi Yanagishita from Tokyo Metropolitan University is advancing the application of anodic porous alumina (APA) in antibacterial technology. By immersing polished aluminium surfaces in an electrochemical cell under specific conditions, the team developed a highly ordered array of porous alumina (aluminium oxide) pillars on the surface. These needle-like structures are precisely designed to kill bacteria, making the treated surfaces highly antibacterial.
The researchers have refined their process, discovering that APA surfaces produced in concentrated sulfuric acid demonstrate significantly enhanced antibacterial properties compared to existing solutions. Importantly, these surfaces were found to be non-toxic to biological cells cultured on them.
Antibiotics are often added to the culture medium in traditional cell cultures to prevent bacterial contamination. However, this approach has critical limitations, including ineffectiveness against antibiotic-resistant bacteria and the risk of fostering more resistant strains due to overuse. APA surfaces eliminate the need for such additives, offering a safer and more sustainable solution for cell culture applications.
Application of revolutionary technologies in medicine
The team’s discovery brings promising advancements to regenerative medicine, where cells are grown in laboratories before being used to repair damaged tissues or organs in patients. Any bacterial contamination in these cells can pose serious risks, especially for individuals who are already unwell. To prevent this, scientists typically rely on expensive, highly sterile environments. However, the team believes their innovative substrates could pave the way for antibiotic-free cell cultures, making growing cells safely in a broader range of settings possible. This breakthrough can transform how patients are treated and significantly expand the scale and accessibility of scientific experiments.
Top image credit: Physics World
Information credit: SciTechDaily
Responses








