

Catalysts play a pivotal role in expediting chemical reactions and enhancing their efficiency. The advancement of new catalytic technologies is crucial for driving the transition towards green energy. The Rice University laboratory led by nanotechnology innovator Naomi Halas has discovered an innovative method for utilising the catalytic capabilities of aluminium nanoparticles. This involves subjecting them to high temperatures while annealing in different gas atmospheres.
About the study
In a study highlighted in the Proceedings of the National Academy of Sciences, researchers from Rice University and their collaborators showcased that modifying the structure of the oxide layer enveloping the particles can significantly alter their catalytic behaviour. These discoveries position aluminium nanoparticles as a versatile tool for diverse applications, ranging from producing sustainable fuels to facilitating water-based reactions.
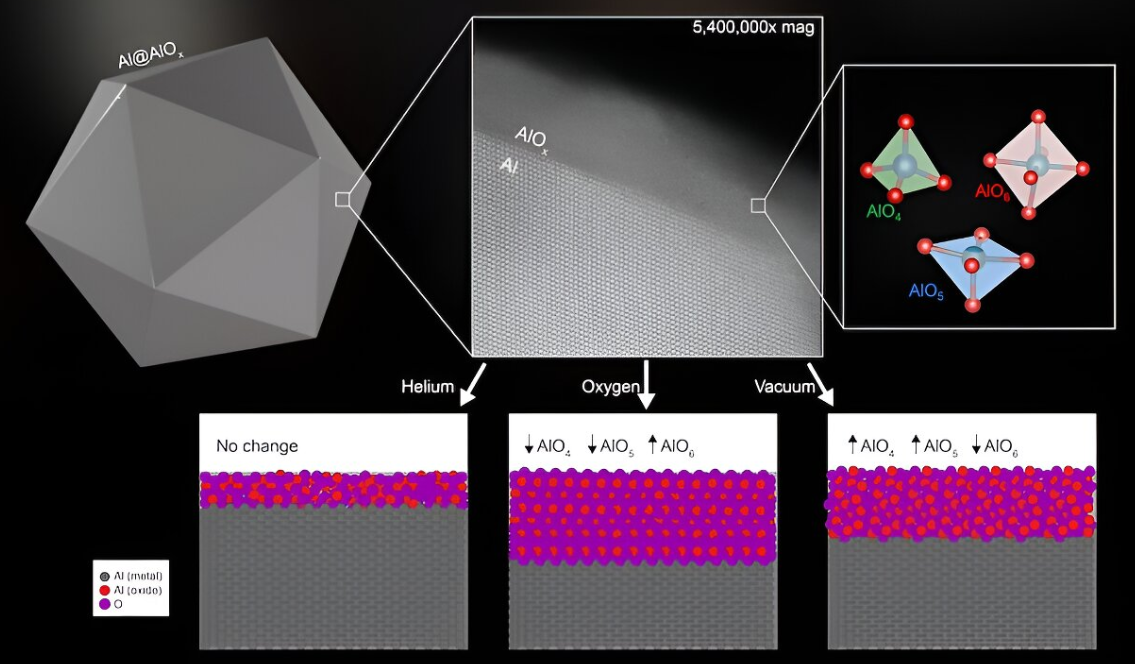
"Aluminum is an earth-abundant metal used in many structural and technological applications. All aluminum is coated with a surface oxide, and until now we did not know what the structure of this native oxide layer on the nanoparticles was. This has been a limiting factor preventing the widespread application of aluminum nanoparticles" said Aaron Bayles, Study Lead Author and Doctoral Alum, Rice University.
Aluminium nanoparticles possess remarkable light absorption and scattering abilities due to surface plasmon resonance. This phenomenon describes the synchronised oscillation of electrons on the metal's surface when it encounters specific wavelengths of light. Like other plasmonic nanoparticles, the aluminium nanocrystal core functions as a nanoscale optical antenna, rendering it an appealing catalyst for light-induced reactions.
"Almost every chemical, every plastic that we use on a day-to-day basis, came from a catalytic process, and many of these catalytic processes rely on precious metals like platinum, rhodium, ruthenium, and others," added Bayles.
"Our ultimate goal is to revolutionize catalysis, making it more accessible, efficient, and environmentally friendly," stated Halas, who holds the title of University Professor, the highest academic rank at Rice.
Enhancing aluminium nanoparticles
The Halas team has directed its efforts towards enhancing aluminium nanoparticles for plasmonic photocatalysis purposes, such as decomposing hazardous chemical warfare agents and efficiently producing commodity chemicals. The newfound ability to modify the surface oxides of aluminium nanoparticles broadens their potential as highly efficient catalysts for converting light into chemical energy.
The properties of the oxide coating on nanoparticles are pivotal in determining their interactions with other molecules or materials. This research delves into the structure of the inherent oxide layer on aluminium nanoparticles. It illustrates that simple thermal treatments can modify this structure, such as heating the particles to temperatures reaching 500 °Celsius (932 °Fahrenheit) in different gases.
“If you are doing a catalytic reaction, the molecules of the substance you’re looking to transform will interact with the aluminum oxide layer rather than with the aluminum metal core, but that metallic nanocrystal core is uniquely able to absorb light very efficiently and convert it into energy, while the oxide layer fulfills the role of a reactor, transferring that energy to reactant molecules,” added Bayler.
“Changing the alumina layer in this manner affects its catalytic properties, particularly for light-driven carbon dioxide reduction, which means the nanoparticles could be useful for producing sustainable fuels,” stated Bayles.
Responses








