您想继续阅读英文文章还
是切换到中文?
是切换到中文?

THINK ALUMINIUM THINK AL CIRCLE

The use of aluminium to bind phosphorus in sediments is a well-established method for mitigating eutrophication in lakes with high nutrient storage. Eutrophication is the process in which a water body is excessively enriched with nutrients, which leads to an overgrowth of simple plant life. The rapid proliferation of algae and plankton often marks this process.

Aluminium posed no threat to aquatic life
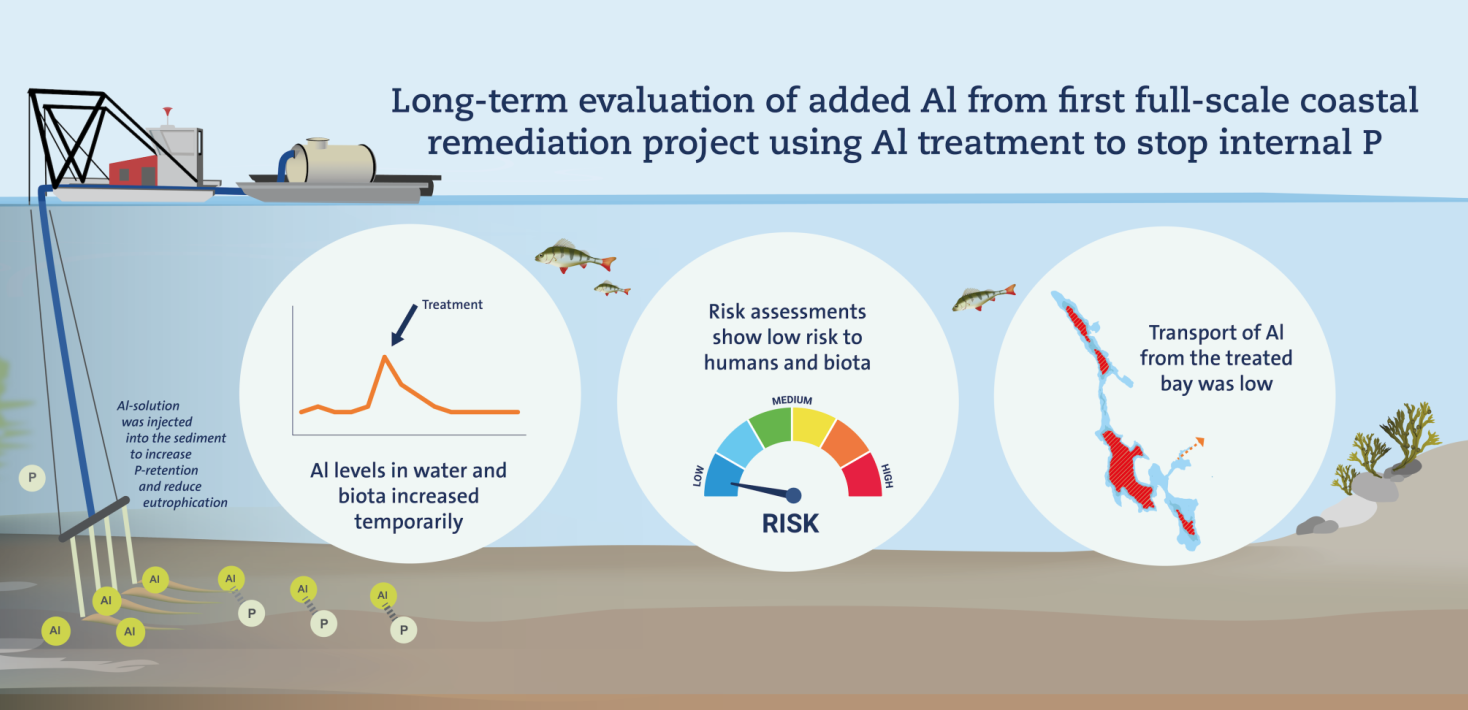
A new study revealed that the aluminium treatment applied to the formerly eutrophic bay, Björnöfjärden, posed no significant threat to humans, animals, or vegetation. While aluminium levels briefly increased in the water, fish, and algae following the treatment, the impact was temporary, and aluminium transport to nearby areas remained minimal.
This technique was tested on a large scale for the first time in Björnöfjärden, located in the Stockholm archipelago, during eight weeks in the summers of 2012-2013 as part of the Living Coast demonstration project.
"Even in the period immediately after the treatment, aluminium levels were 17 times lower than would be risky for human consumption," said researcher Linda Kumblad. "For example, bread or cereals normally contain much higher levels of aluminium than the fish in Björnöfjärden.
About the treatment
The treatment quickly reduced phosphorus levels in the water, leading to environmental improvements in the bay. Researchers have examined how aluminium levels in the water, fish, and algae changed during and after the treatment, assessing potential risks to humans and local ecosystems.
The analysis, which includes ten years of data from Björnöfjärden and a control bay, revealed a 50 per cent increase in aluminium concentration in the surface water following the treatment. However, the concentration had returned to pre-treatment levels by the following year.
"The aluminium was applied directly into the bottom sediment to bind the dissolved phosphorus there. But the dose was adjusted to also bind the phosphorus to be released in the coming years", explained Emil Rydin, researcher at the Stockholm University Baltic Sea Centre and one of the authors of the study.
The researchers also assessed the amount of aluminium transported from Björnöfjärden to the neighbouring bay, Nämdöfjärden. In 2013, the estimated transport was 1 tonne, representing approximately 3 per cent of the aluminium applied during the treatment.
Source - Stockholm University
Analysis of the aluminium level
Aluminium levels were also analysed in bladderwrack and perch samples from the bay. No clear pattern emerged in the perch's muscle tissue. Still, there was a noticeable increase in aluminium in the liver and gills following the treatment, which quickly returned to normal levels.
Although no pre-treatment measurements were taken for bladderwrack, post-treatment levels in Björnöfjärden were higher than those in the control bay. However, a few years later, the concentrations had decreased to similar levels found in comparable bays, while the control bay's levels remained slightly lower.
Research showed lower risk on bladderwrack
Concerns have occasionally been raised about the potential harm aluminium treatments in lakes and seas might pose to aquatic life. This stems partly from evidence that aluminium released from soil during acidification can damage fish gills. However, the new study indicates that the risk of adverse effects on bladderwrack or perch in Björnöfjärden was very low.
"The fact that the transport was so low and that the concentration in the water fell rapidly shows that the method of adding aluminium directly to the bottom sediments was effective. The aluminium stayed in the sediments and bound phosphorus, as intended," added Emil Rydin.
According to the risk assessment, aluminium levels posed no significant risk to individuals consuming fish from Björnöfjärden or drinking desalinated water sourced from the area. The researchers conclude that the Björnöfjärden project demonstrates that aluminium treatment can effectively reduce eutrophication, even in coastal regions.
"Björnöfjärden is not acidified - the pH is stable between 6 and 8 - and there are also relatively high levels of dissolved organic carbon (DOC) and other substances that reduce toxicity. For aluminium to pose a risk under these conditions, the concentration would have to be many times higher than what was measured in Björnöfjärden during the Al-treatment," stated researcher Linda Kumblad, another author of the study.
Responses








