您想继续阅读英文文章还
是切换到中文?
是切换到中文?

THINK ALUMINIUM THINK AL CIRCLE

The latest type of aluminium ion battery which is flexible and has the same amount of energy as a lead-acid battery or even a nickel-metal hydride battery has been developed by researchers at the Stanford University in California.
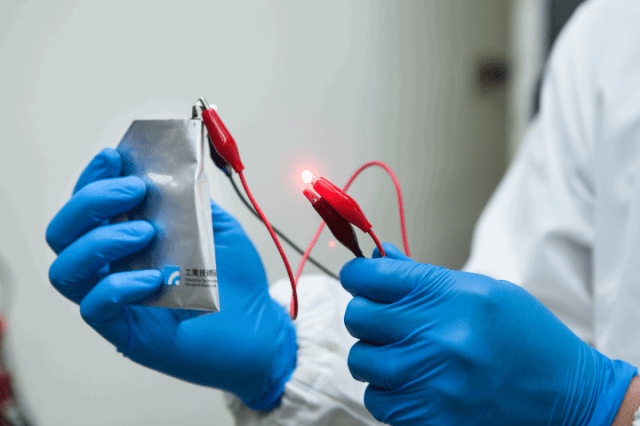
The key dominance of this latest type of battery is that it can charge itself in a minute. This battery has another advantage; it possesses a much longer life cycle than the existing battery technologies of today. Furthermore, the cost of the battery is low and it is stable. These findings were declared by Professor Hongjie Dai of the Stanford University and he also stated that such properties of this new kind of battery along with its stability make it a contender for grid-scale storage. This battery can also be used to power wearable devices.
Aluminium ion batteries come up with multiple numbers of distinct advantages over lithium-ion batteries. One of the crucial reasons is aluminium is readily available in abundant supply, it is a cheaply available material for the battery. Aluminium also tends to be less reactive, which also lessens the chances of batteries exploding as they are less flammable. Researchers demonstrated the properties of their newly developed battery by drilling a hole in the batteries while they were still working, and the batteries didn’t explode or catch fire.

The researchers said: “If we pay attention to the chemistry of aluminium, we will know it has three valence electrons as compared to lithium that has only one valence electron. Due to this property of aluminium, the charge-discharge reactions can transfer three electrons per atom. This translates into the possibility that an aluminium ion battery can carry also three times the energy as compared to its lithium-ion counterpart, and that too in a much lighter and a smaller avatar.”
On the contrary, it has to be noted that scientists have been trying to develop an effective aluminium ion battery for more than three decades without any success. In most of the previous designs, solid aluminium anodes have been used. For a cathode, manganese oxide and doped polymers have been used. However, the best of such batteries have certain inherent disadvantages such as low discharge voltages, the shorter cycle life of fewer than 100 cycles and don’t have the proper capacity to store a large amount of energy. Also, the cathode used for such batteries used to disintegrate quickly.
Professor Dai and his colleagues proclaim that they have conquered all such drawbacks in the aluminium ion battery. They claim to have found an efficient cathode that works much better. This cathode is in the form of flexible 3D graphite foam, which is a porous substance and is a lightweight and sponge-like carbon material that they have synthesised in their laboratory. Due to the porous structure of this cathode, it can hold a large number of aluminium electrons in its pores. Besides, due to the porous nature of this cathode, the electrons can move quickly, due to which the battery can be charged and discharged in a short period. The researchers used aluminium in the form of an anode and packed the graphite in a flexible pouch containing ionic liquid electrolyte. This design of the battery performed well on all the parameters.

Besides, the 40 watt-hours per kilogram energy density of this aluminium ion battery are comparable to lead-acid and nickel batteries. The advantage of this new type of battery sets in the fact that it has a much longer life cycle than its competitors. This type of battery lasted for 7,500 charge cycles without exhibiting any loss in capacity. In comparison, lithium-ion batteries last only for 1,000 charge cycles.
Professor Dai and his team are working to make a cheap ionic electrolyte for this aluminium ion battery that is cheaper and can compete cost-wise in the market. They are also working to increase the energy storage capacity of the graphite cathode. Multiple companies have shown interest in the aluminium ion battery technology.
Responses








